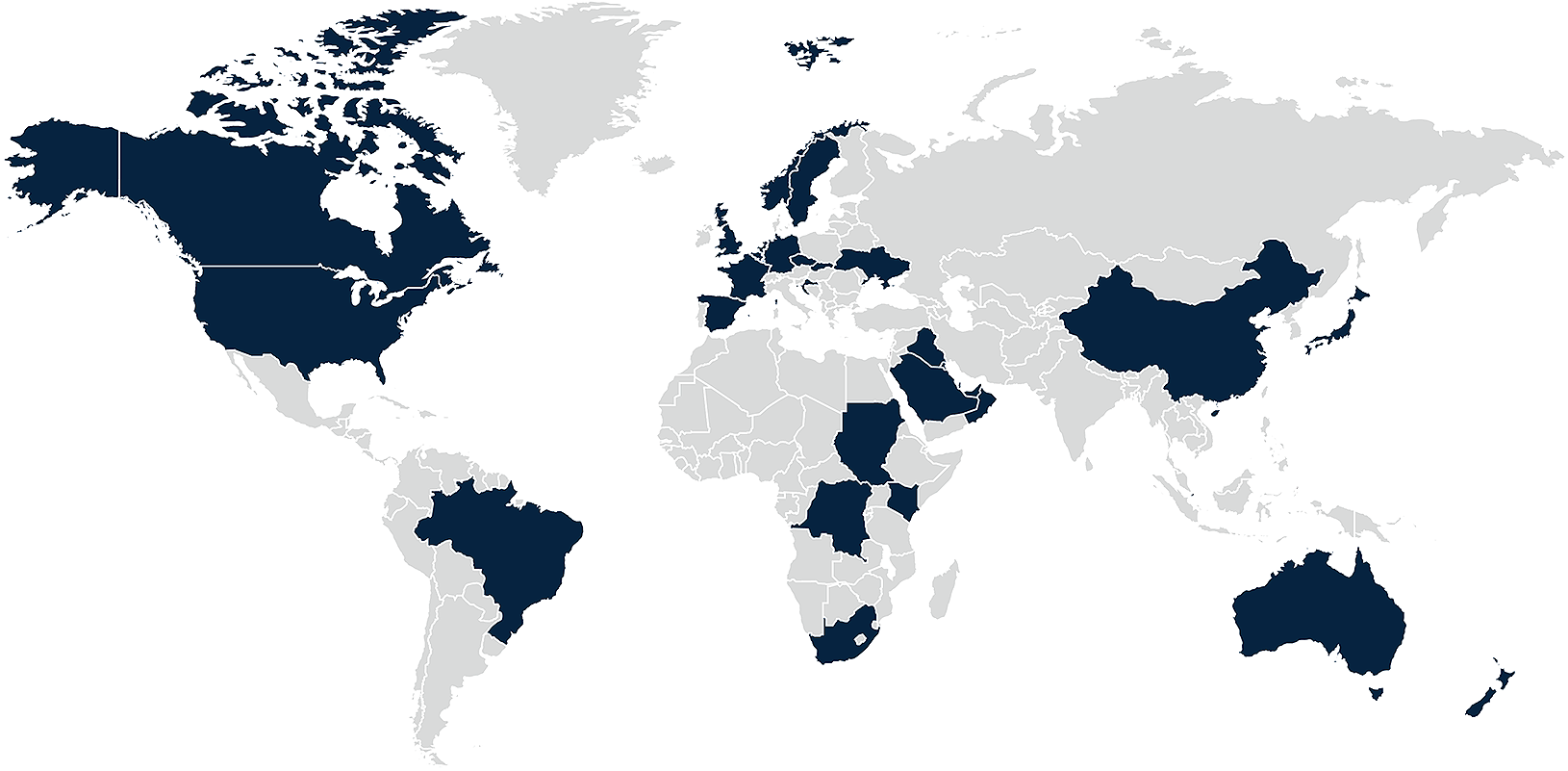
Explore compliance requirements for pharmaceutical companies engaging healthcare professionals around the world

Pharmaceutical companies rely on the knowledge and expertise of healthcare professionals for purposes such as scientific discussions, clinical trial design, new product development, and information and promotion of new treatments. All such interactions are subject to strict legal and regulatory requirements in most jurisdictions around the world.

DLA Piper’s global Life Sciences team has prepared this interactive comparison site to give you an overview of global compliance requirements and restrictions applying to pharmaceutical companies wishing to interact with healthcare professionals in over 35 jurisdictions around the world. The site outlines the limitations set by local rules, including law, guidance and codes of conduct in relation to hospitality offered by pharmaceutical companies to healthcare professionals. The site also enables you to compare several jurisdictions easily and quickly.
The site is not a substitute for legal advice. Should you require further information about the topics covered in the guide, please contact us here.
Explore
By Country