Privacy requirements for clinical trials: a cross-border guide
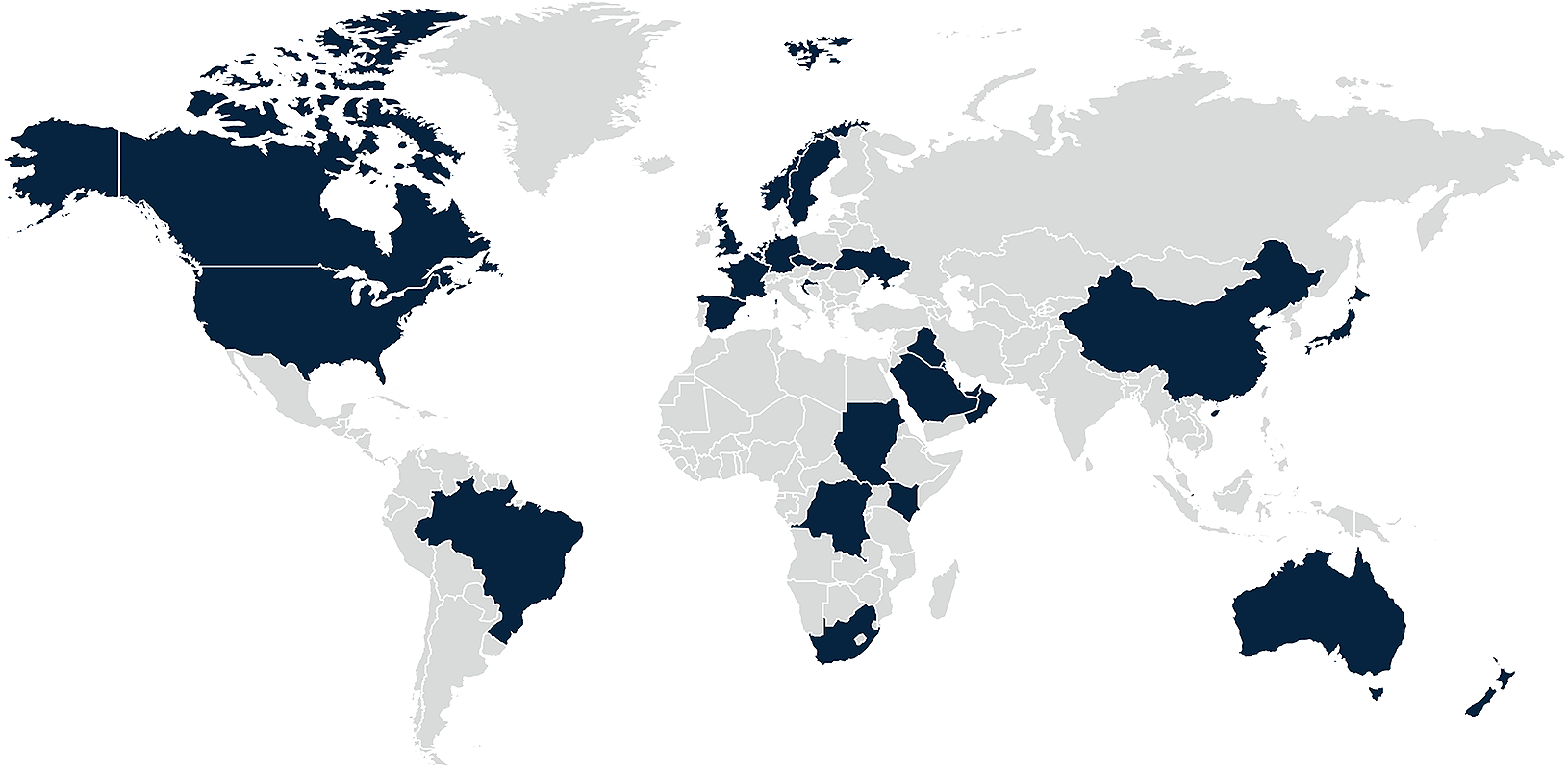
Clinical trials often take place on a cross-border basis, involving sites in a number of different jurisdictions. However, experience shows that it can be difficult to understand and manage the privacy requirements for cross-border trials. In a large part, this is due to differences in local law and interpretation relating to the interplay between privacy laws and clinical trials. This is the case even within the EU: although the GDPR is directly applicable in all member states, there are often differences in the way these countries, and their national privacy and medicines regulators, interpret and apply the Regulation to the context of clinical trials.

Explore
By Country